MOBILE PHONE READER: ROTATE TO HORIZONTAL POSITION FOR A BETTER READ
Photoredox catalysis has emerged as a powerful and sustainable strategy in organic synthesis, utilizing light energy to drive redox reactions. This approach offers significant advantages over traditional methods, such as milder reaction conditions, greater selectivity, and a reduced environmental impact by minimizing waste and energy consumption.
In photoredox catalysis, the catalyst, typically a transition metal (TM) complex or polyaromatic organic molecule, absorbs light (usually ultraviolet or visible) and undergoes photoexcitation. Such a process basically consists in promoting an electron from a bonding or nonbonding orbital (highest occupied molecular orbital – HOMO) to an antibonding orbital (lowest occupied molecular orbital – LUMO). In TM complexes, this means an electron transfer from the metal to the ligand and therefore is also called Metal to Ligand Charge Transfer (MLCT). Therefore, the photoexcitation generates simultaneously a higher energy single occupied molecular orbital (SOMO) and a corresponding lower energy SOMO in the same species, which makes it capable of selectively engaging in redox reactions with substrates. In other words, the photocatalyst is a versatile species, capable of activating substrates by single electron reduction (oxidative quenching) or oxidation (reductive quenching). The choice of which pathway is the most thermodynamic feasible is dictated by the matching of redox potentials between the photoexcited catalyst and the redox active species present in the reaction medium.

Although an analysis of the redox potential is necessary to estimate a photocatalytic reaction outcome, a brief analysis of the structure of some neutral and charged organic molecules can be enough to classify them as electron donors or acceptors.
Transition metal complexes, such as those of ruthenium and iridium are widely used as photocatalysts due to their ability to absorb visible-light and reach excited states with a lifetime enough to engage in intermolecular redox processes. However, in recent years, organic photocatalysts have gained significant attention due to their promising potential. Organic photocatalysts offer several advantages, including cost-effectiveness, environmental benignity, and the ability to be finely tuned to absorb visible light more efficiently. They also often exhibit better selectivity for specific reactions compared to metal-based catalysts.

What we want to do:
One of our research goals is to develop new synthetic protocols based on cyanoarene photocatalysts. The investigations will not only focus on explore well-estabilished catalysts but also on designing new ones. Moreover, with the aim of contributing to advances in low-energy photocatalysis, protocols based on red-light sensitive photocatalysts are also being studied.
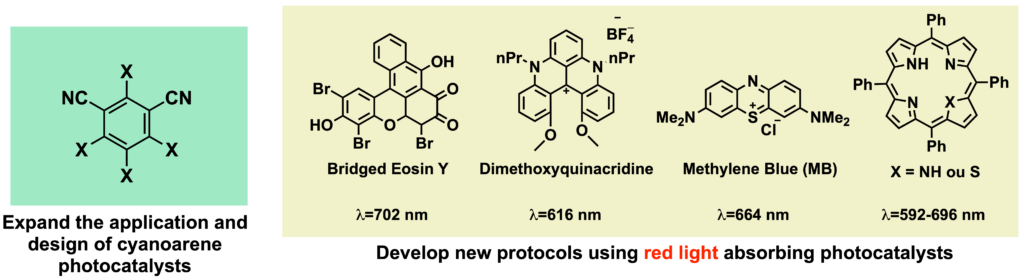
References:
Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 17, 10075–10166
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis. Beilstein J. Org. Chem. 2020, 16, 1163–1187
A Toolbox Approach To Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor–Acceptor Cyanoarenes. J. Am. Chem. Soc. 2018, 140, 45, 15353–15365
