MOBILE PHONE READER: ROTATE TO HORIZONTAL POSITION FOR A BETTER READ
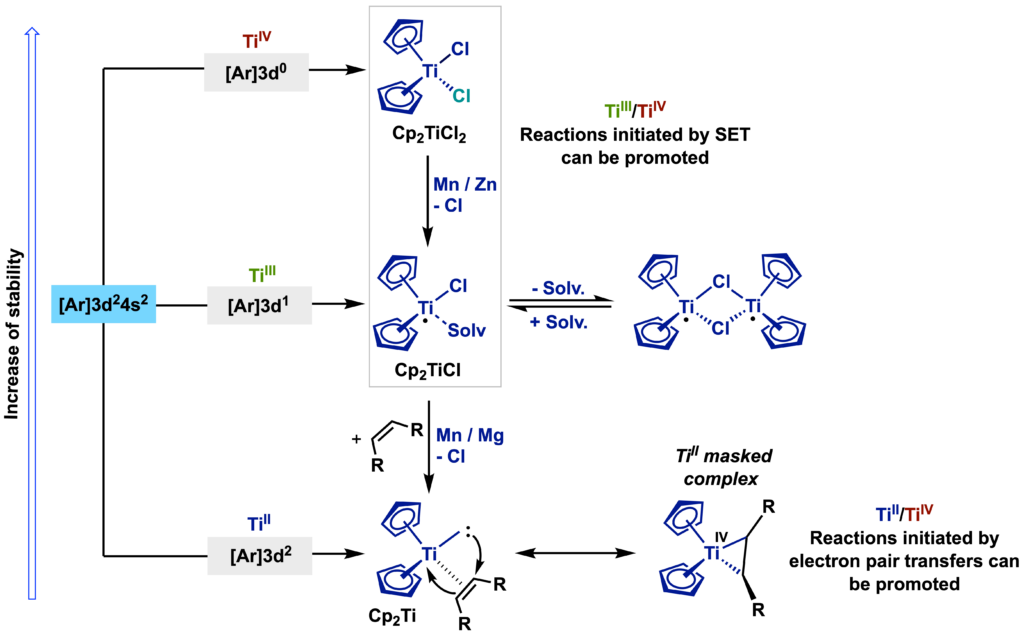
Titanium is the second most abundant transition metal, only surpassed by iron, and the ninth most abundant element in the Earth’s crust. It is low cost and low toxicity, and its biocompatibility is well known. The electronic configuration of titanium is [Ar]4s23d2 and its complexes can adopt oxidation states ranging from +2 to +4, with Ti(IV) being the most thermodynamically stable because it has the electronic configuration of argon
Ti(III) complexes are easily accessible through the treatment of the Ti(IV) complex with reducing agents (Mn, Zn, or Mg) and can be stabilized by the presence of bulky ligands and good s- and p-donors, with Cp (cyclopentadienyl) ligands being the most widely used. Ti(II) complexes are accessed indirectly, being transferred in solution to the reaction medium in which they will act. In these cases, Mg is generally used as a reducing agent, and in addition to the Cp ligands, an additional strong p-acceptor ligand (alkene or alkyne) is necessary, giving rise to Ti(IV) metallacycles called “masked” Ti(II) complexes. In summary, titanium complexes are capable of promoting redox processes involving the transfer of one electron [Ti(III)/Ti(IV)] or two electrons [Ti(II)/Ti(IV)].
Important landmarks
The synthesis of Cp2TiCl (2) from Cp2TiCl2 (1) was first reported by Wilkison in 1955, and it was isolated as a binuclear complex (3), characterized as a green crystalline powder (Scheme 2A). The behavior of these species in solution was only carefully characterized years later by Skrydstrup, who demonstrated that 2 is coordinated to a solvent molecule (2.S) and in equilibrium with 3. The Lewis acid nature and oxophilicity of titanium complexes cause them to bind strongly to carbonyl compounds, epoxides, alcohols, and other species containing unpaired electron pairs. In the case of 2, this behavior is facilitated by the existence of an empty orbital in its valence shell, which is composed of 15 electrons (paramagnetic), which also causes it to present a half-filled d orbital. These characteristics, associated with the reducing character of 2 (Ered = –0.83 V vs Fc+/Fc]), allow this complex to promote the homolytic cleavage of an adjacent C-X/C=X bond, through an intra-coordination sphere electron transfer. Based on this behavior, between the late 1980s and early 1990s, Nugent and Rajan Babu investigated the application of 2, still stoichiometrically, in the radical opening followed by functionalization or deoxygenation of epoxides, so that this complex came to be popularly called the “Nugent-Rajan Babu reagent”.
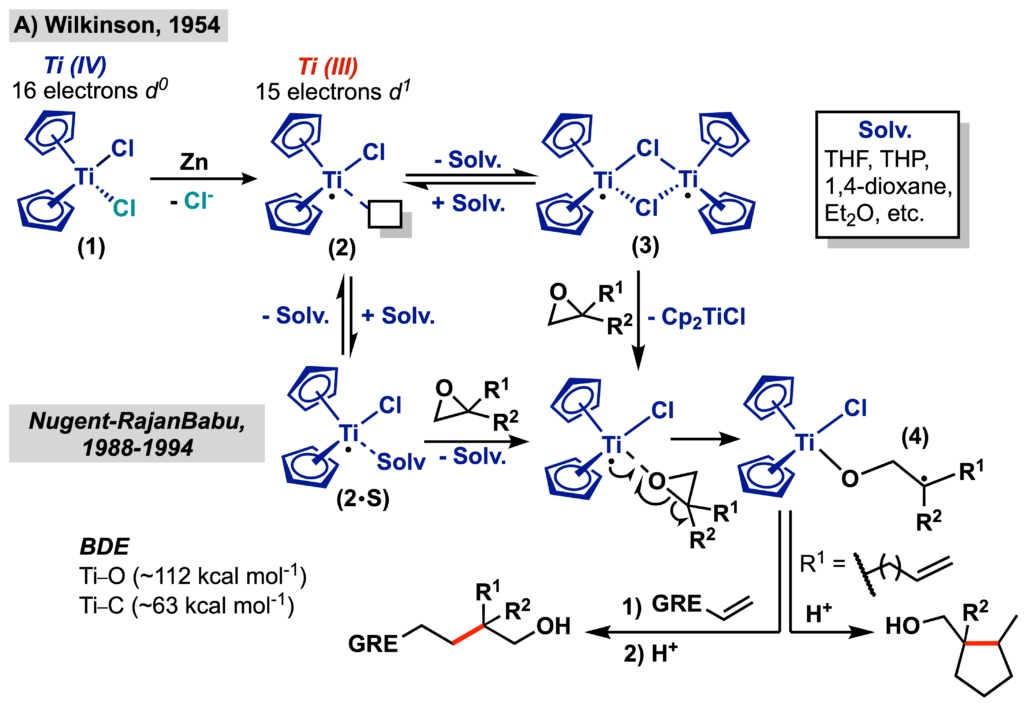
These seminal works set the precedent for the application of Cp2TiCl in total synthesis of natural products, but it was not until 1998 that the first catalytic radical functionalization of epoxides was described by Gansäuer, making this approach even more attractive from a synthetic point of view. In the strategy developed by Nugent and Rajanbabu, the titanium complex remains bound to the reaction intermediates until the product is formed, requiring an acid treatment in the isolation step, which then justified the stoichiometric use of 2. However, Gansäuer discovered that 2,4,6-collidine hydrochloride (Coll•HCl) was acidic enough to promote the cleavage of the Ti(IV)-O bond, whereas the collidine generated as a byproduct of this process was sterically congested enough to have its coordination to the titanium complex disfavored. This then allowed the generation of the product and the regeneration of 1.
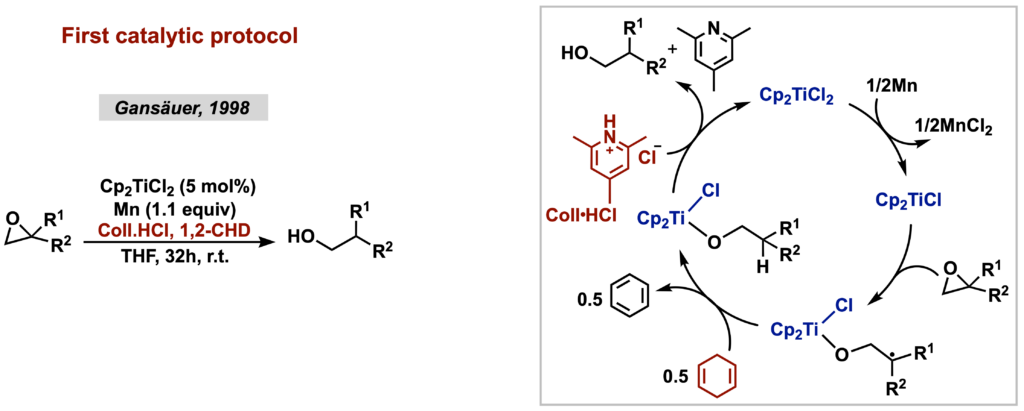
The use of (over)stoichiometric amounts of strong metallic reducing agents – generally insoluble in the organic solvent used – and acid additives, also in over-stoichiometric amounts, reduces the applicability of titanium redox catalysis when aspects related to tolerance towards some functional groups, scale-up, translation to continuous flow and reduction of waste generation are considered. In view of this, photo- and electrocatalytic strategies have recently emerged as formidable alternatives to overcome some of these limitations. When applied to titanium redox catalysis, they can be used both to improve and expand the scope of previously reported transformations, and to develop new synthetic methodologies using substrates and radical sources incompatible with traditional reaction conditions.
Gansäuer’s group also pioneered the photoredox catalysis of titanium, reporting the first example of opening and opening-followed intramolecular radical arylation of epoxides using an iridium complex as a photocatalyst. Soon after, Shi and co-workers reported another protocol for the opening followed by spirocyclization of epoxides, in this case using 4CzIPN as the catalyst. These works were followed by a series of contributions, most of which focused on allylation and propargylation reactions, on the difunctionalization of butadiene and allenes through Barbier-type reactions using aldehydes as electrophiles, and on the opening and functionalization of cyclic oximes.

What we want to do:
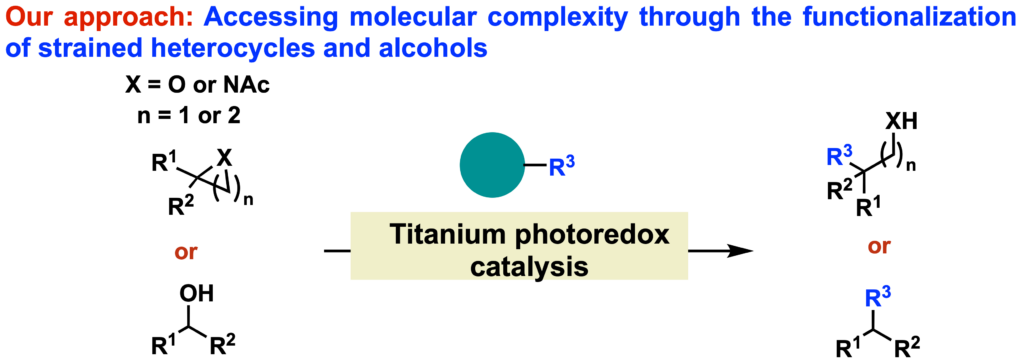
Inspired by those precedents, our group will explore new reactivities promoted by titanium photoredox catalysis, focusing on the ring opening and functionalization of strained heterocycles (epoxides, aziridines, azetidines and oxetanes) and on the direct desoxifunctionalization of alcohols. Besides accessing molecular complexity, we aim to design new tools that can be employed at different stages of the synthetic plan.
References:
Advances and Applications in Catalysis with Earth-Abundant Metals. Org. Process Res. Dev. 2023, 27, 1157.
Titanium catalysis for the synthesis of fine chemicals – development and trends Chem. Soc. Rev. 2020, 49, 6947–6994.
Recent Advances in Titanium Radical Redox Catalysis J. Org. Chem. 2019, 84, 14369–14380.
Titanium radical redox catalysis: Recent innovations in catalysts, reactions, and modes of activation. Chem 2022, 8, 1805.
Cp2TiIIICl Catalysis in a New Light. ChemPhotoChem 2020, 4, 659–663.
Titanium in photocatalytic organic transformations: current applications and future developments Org. Biomol. Chem., 2024, 22, 6650.
