Advanced Oxidation Processes (AOP)
When talking about compromising water resources, concerns about the so-called micropollutants or xenobiotics have been growing, arousing the interest of society and the scientific community. Many of these compounds are classified as contaminants of emerging concern (CECs), such as pesticides, antibiotics, analgesics, hormones, organochlorines, phenols, etc. The cause of the pollution of water resources is the inadequate disposal of untreated or partially treated domestic and industrial sewage, causing different impacts on surface and underground water bodies, in terms of size and complexity, depending on the composition of the waste released. Little is known about the impacts of these pollutants on the environment and human health. Some drugs may favor the development of multi-resistant strains of pathogenic bacteria, in addition to affecting the endocrine system of fish and exerting toxic effects on algae and invertebrates.
Water and wastewater treatment facilities have implemented processes not capable of completely removing such pollutants. Conventional treatments (flocculation, adsorption on activated carbon, filtration, incineration, biological treatment, etc.) are associated with technical or economic limitations. For example, with the exception of the last two, physical-chemical processes concentrate pollutants, most of the time without degrading them, reducing their volume, but creating the problem of final disposal.
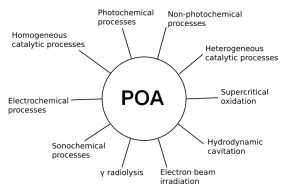
More restrictive legislation regarding the presence of micropollutants in water and wastewater, combined with social demands regarding the impacts of these substances on the environment and human health, have fostered the development of more efficient treatment technologies, including oxidation and photo-oxidation processes, collectively called Advanced Oxidation Processes (AOP). Such technologies involve reactive oxygen species (ROS), which make it possible to degrade organic pollutants with different chemical structures and functional groups to less toxic and/or more easily biodegradable substances, leading, in some cases, to complete oxidation to carbon dioxide, water, and inorganic compounds, i.e., components of the heteroatoms other than oxygen. In many cases, however, mineralization is not complete due to the recalcitrance of transformation products (TPs).
An example of ROS is the hydroxyl radicals (HO•), species with a strong oxidizing character, which react with most organic substances with low selectivity and extremely high specific reaction rates (108-1010 L mol-1 s-1). The main reaction mechanisms of HO• radicals with organic pollutants involve hydrogen abstraction from aliphatic carbons, electrophilic addition to unsaturations and aromatic rings, and electron transfer.
Photochemical degradation promoted by solar radiation and the action of intermit reactive species (hydroxyl radicals, HO•; singlet oxygen, 1O2; and chromophoric organic matter in the triplet state, 3CDOM*). Such species are generated from the adsorption of light by other chemical species present in natural water bodies, such as chromophoric dissolved organic matter (CDOM), as well as from reactions involving ions present in water bodies such as nitrite (NO2–) and nitrate (NO3–) (indirect photolysis). They have an important role in the photochemical destination of emerging micropollutants in natural waters.
Other AOP applications includes, for example, sugarcane juice clarification and tooth whitening.



 Imprimir
Imprimir