O material genético contido em nossas células é surpreendentemente reativo, apesar de ter a função de armazenar informação. O DNA reage com diversos metabólitos celulares, como aldeídos e espécies reativas de oxigênio, diferentes agentes químicos e físicos ao nosso redor, como componentes do cigarro, radiação ultravioleta do sol ou radioatividade, além da própria água, de forma que o DNA precisa ser constantemente reparado da maneira mais fiel possível para evitar o acúmulo de mutações que levam ao câncer, envelhecimento e neurodegeneração.
Nós estudamos as vias moleculares de sinalização e reparo destes danos ao DNA, utilizando técnicas de biologia molecular e celular, por exemplo Western Blotting, microscopia de fluorescência, tecnologia CRISPR/Cas9 e ensaios funcionais. Dentro dessa linha de investigação, os projetos em desenvolvimento no laboratório se encaixam basicamente em três áreas de interesse:
ADP-ribosilação e reparo de DNA
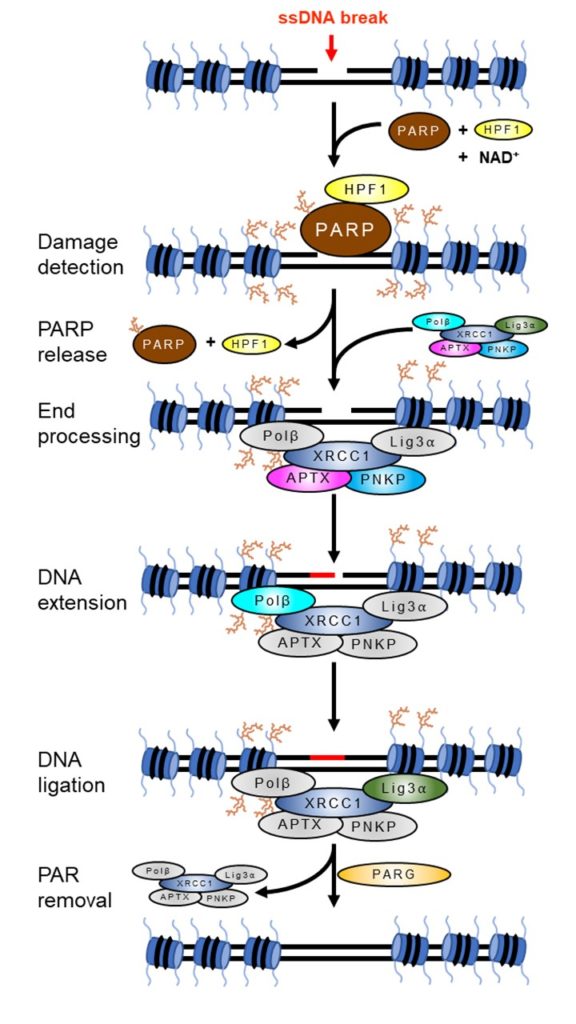
ADP-ribosilação é uma modificação pós-traducional de proteínas com importante função na sinalização da presença de danos ao DNA, remodelamento da cromatina e recrutamento de fatores de reparo de DNA para o local da lesão, além de um tipo de morte celular programada chamada parthanatos. Esta modificação é catalisada por uma classe de enzimas chamadas poli(ADP-ribose) polimerases (PARPs), diversas das quais são ativadas por danos ao DNA. Centenas de proteínas são modificadas por ADP-ribose, outras tantas reconhecem e se ligam a esta modificação, e outras mais são envolvidas na remoção deste “sinal” após a conclusão do reparo. Nós buscamos entender os mecanismos moleculares pelos quais cada um desses eventos sinaliza a presença de danos ao DNA e acelera o seu reparo. (Figura 1)
Para saber mais, dê uma olhada no nosso artigo:
Hoch NC, Polo LM. ADP-ribosylation: from molecular mechanisms to human disease. Genet Mol Biol. 2019 Dec 13;43(1 suppl 1):e20190075.
Reparo de DNA e doenças genéticas raras
Diversas síndromes genéticas raras são causadas por mutações em genes envolvidos na sinalização e reparo de DNA. Estas doenças são geralmente caracterizadas por predisposição ao câncer, envelhecimento precoce, imunodeficiência e/ou problemas neurológicos, como neurodegeneração ou microcefalia. Nós estudamos as consequências moleculares das mutações encontradas em pacientes para entender os mecanismos que contribuem para estas patologias e identificar possíveis alternativas terapêuticas.
Veja nosso artigo:
Funções da ADP-ribosilação em outras vias de sinalização
Um terceiro tema de interesse do laboratório, que vem ganhando espaço especialmente durante e após a pandemia de COVID-19, é a função da ADP-ribosilação de proteínas em resposta a outros processos, como a resposta celular a infecções virais. Alguns vírus, incluindo o coronavírus, expressam uma enzima cuja função é hidrolisar (ou seja remover) a ADP-ribosilação de proteínas, que é catalisada por enzimas do hospedeiro como parte da resposta antiviral das células. Nesse contexto, buscamos entender como a ADP-ribosilação contribui para a resposta imune, qual a função desse domínio viral durante a infecção e se a inibição dessa enzima viral pode ser uma alternativa terapêutica.
Para saber mais, dê uma olhada no nosso artigo:
-
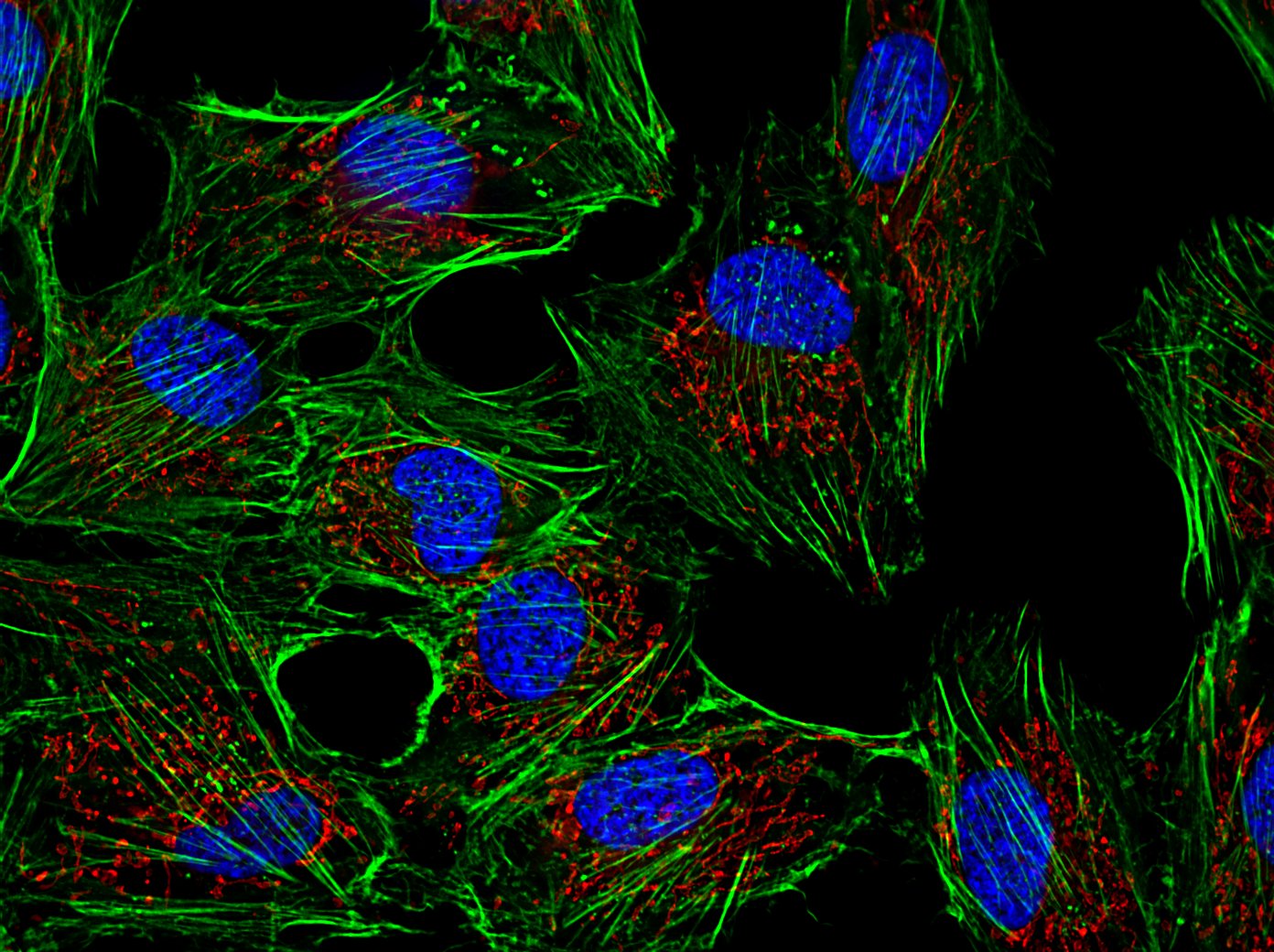
- Microscopia de fluorescência de células humanas em cultura. Azul: DNA no núcleo da células, verde: filamentos de actina, vermelho: mitocôndrias.
-
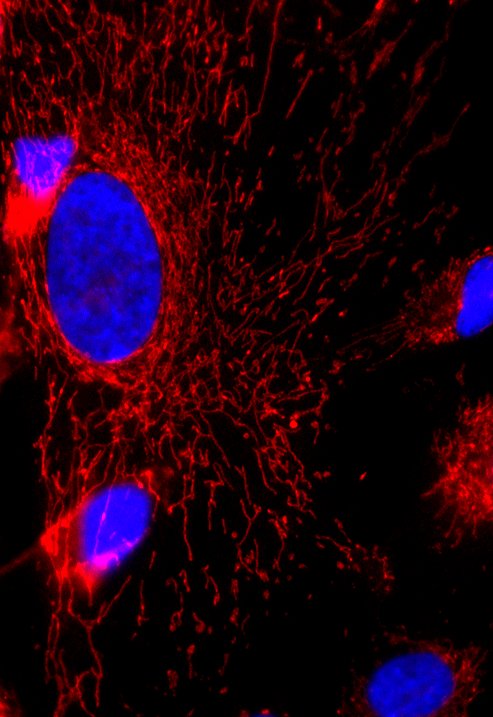
- Microscopia de fluorescência de células humanas em cultura. Azul: DNA no núcleo da célula, vermelho: mitocôndrias.
-
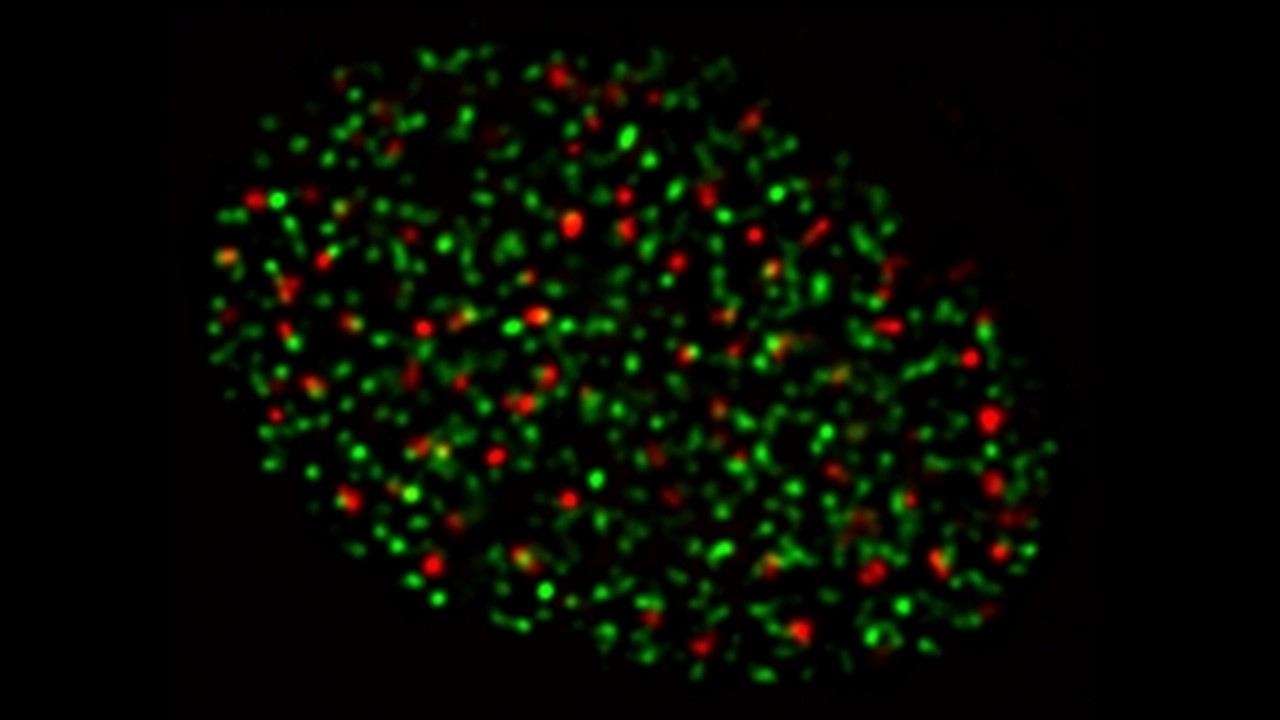
- Microscopia de fluorescência do núcleo de uma célula humana durante a fase S do ciclo celular. Verde: regiões do núcleo em que há replicação do DNA, vermelho: fosforilação da histona H2AX, um marcador de danos ao DNA.
-
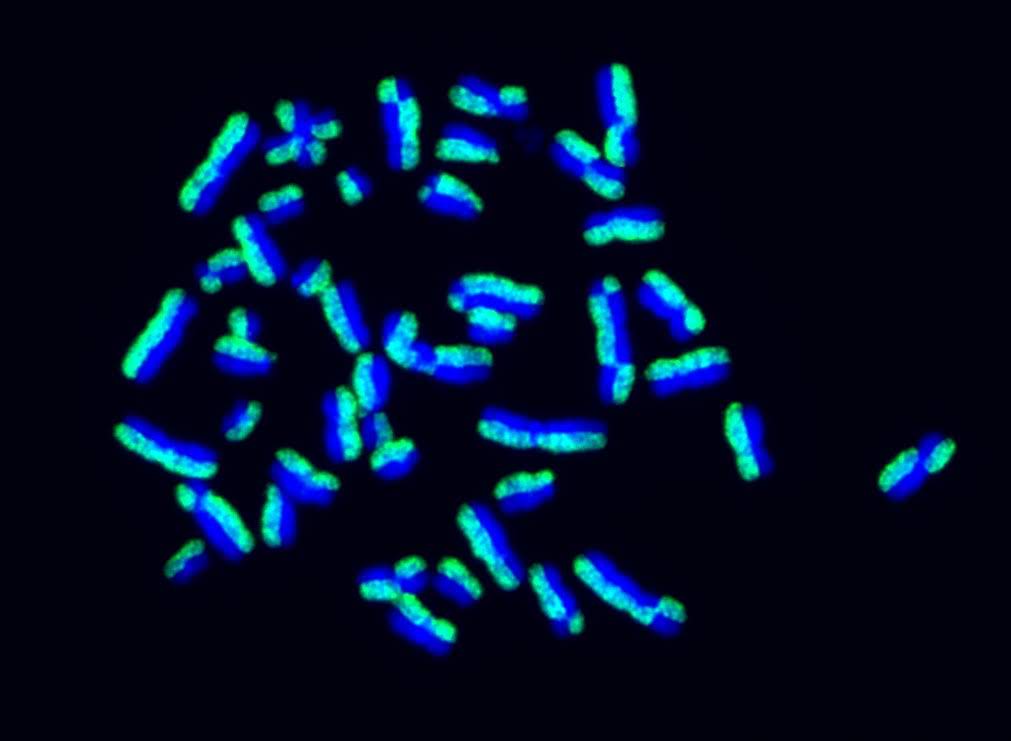
- Microscopia de fluorescência de um ensaio de sister chromatid exchanges. Cromossomos de uma célula humanas estão marcados de forma que uma cromátide irmã seja visualizada em verde e a outra em azul. Em resposta a danos ao DNA, pode haver troca de informação genética entre as cromátides-irmãs pela via de recombinação homóloga, levando a trocas recíprocas no padrão de coloração dos cromossomos.
-

- Ensaio cometa, que mede danos ao DNA por migração do DNA nuclear em gel de agarose. DNA intacto compõe a “cabeça”, enquanto fragmentos de DNA danificado migram mais rápido e formam a “cauda” do cometa.
-
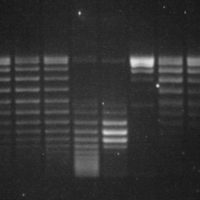
- Gel de agarose de um ensaio de atividade de topoisomerase. DNA plasmidial superenovelado (poço 2) migra mais rápido do que DNA relaxado (poços subsequentes).
-
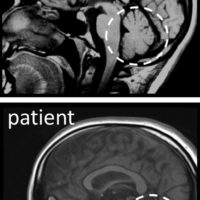
- Ressonância magnética de crânio de um indivíduo normal e de um paciente com degeneração do cerebelo (indicado por um círculo), causada por mutações em um gene de reparo de DNA.
-
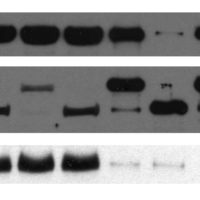
- Imagem representativa de um western blot, demonstrando diferenças entre as amostras, tanto no tamanho quanto abundância das proteínas detectadas.
