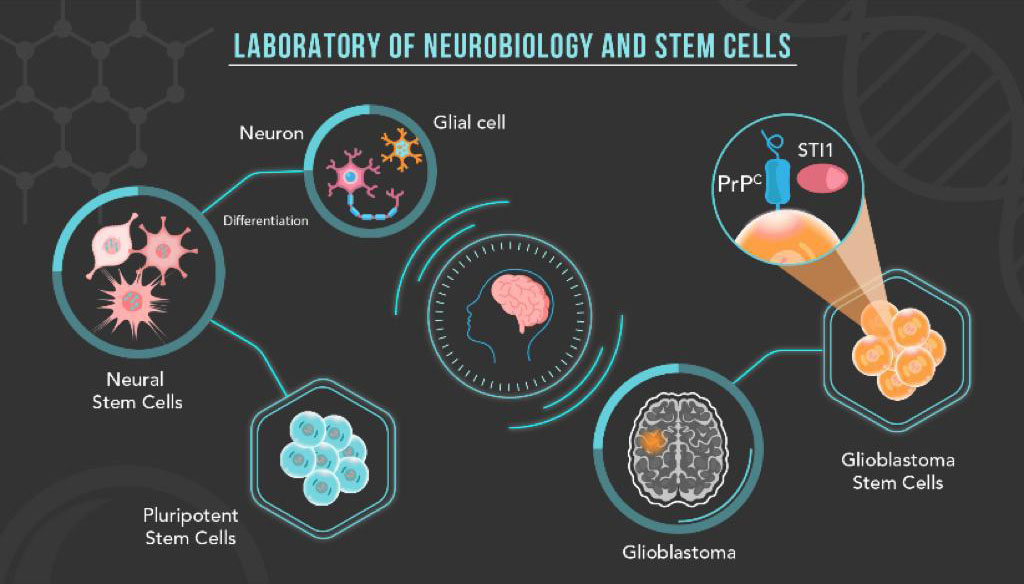
Our research is focused on the study of cellular prion protein (PrPC) and its partners in the biology of pluripotent, neural and tumor cells. PrPC isoforms are related both to important neurodegenerative diseases and to the modulation of different mechanisms that govern neural plasticity. Along with its main ligand, the co-chaperone stress inducible protein 1 (STI1), PrPC orchestrates processes such as neuritogenesis, neuroprotection, memory consolidation, in addition to self-renewal and proliferation of neural stem cells. Therefore, we seek to understand the function of the PrPC-STI1 complex and associated factors at the interface between pluripotent cells and different neural models (neural stem cells, neurons, glial cells), contributing to the knowledge of the importance of this complex in the biology of severe brain tumors, such as glioblastoma.
The current research projects are divided in two main topics:
Pluripotent Stem Cells
Pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotency stem cells (iPSCs), correspond to in vitro models used to mimic early development. Understanding the mechanisms that regulate the intrinsic capacity of these cells of self-renewal and differentiation in all cell types is a way of studying the processes of development, cell differentiation and specialization, in addition to being fundamental to explore their therapeutic potential. Driven by the previous discovery that complete knockout of STI1 leads to mice early degeneration, with the molecular mechanisms that result in this phenotype still unknown, currently our group seeks to elucidate the importance of this protein and its main partners in mammalian initial development and stemness maintenance, using mice ESCs expressing different levels of STI1 as a model.
Glioblastoma Stem Cells
Glioblastoma (GBM), the most aggressive and frequent form of malignant brain tumor, contains a subpopulation of tumor cells, called glioblastoma stem cells (GSCs), essential for tumor maintenance, invasion and resistance to therapy. PrPC is enriched in this subpopulation, being co-expressed with conventional GSCs markers. The loss of PrPC function in CTGs results in the inhibition of self-renewal, proliferation and the ability to form tumors. Our research proposes to understand the role of PrPC and its main partners, for example STI1, as key regulators in the maintenance of stemness in CTGs, and from the knowledge generated to propose the development of new strategies for the treatment of glioblastoma.