

Profa. Dra. Ana Paula Lepique
 Título: Associated Professor
Título: Associated Professor
Laboratory: Immunomodulation
Telefone: +55 (11) 3091 7376
E-mail: alepique@icb.usp.br
Lattes: http://lattes.cnpq.br/0808439926941946
Research interest:
Tumor microenvironment and its effects on the immune system.
Interests and projects:
Cancer is a chronic disease, where a complex tumor microenvironment changes with time due to alterations in tumor cells and modulation of immune responses. We have been interested in understanding how signals generated in the tumor microenvironment can modulate local and systemic immune responses. We investigate the role of metabolites, cytokines, extracellular vesicles in experimental models as tridimensional cell cultures and also in patients’ samples. Moreover, we investigate key signaling pathways involved in carcinogenesis, with important roles in both tumor cells and leukocytes. As examples, we can mention STAT3, SET/PP2A, PI3K/Akt. Our research has been focused on cervical cancer, ovarian cancer and oropharyngeal cancer.
We have been working with the hypothesis that understanding local and systemic effects of the tumor on the immune system may help in design of therapeutic protocols that may increase the efficacy of therapeutic vaccines, anti-checkpoint blockade antibodies or even chemo and radiotherapy.
A few results:
In the recent years we have worked on the characterization of the leukocyte populations in cervical tumors and precursor legions. We showed that the most abundant leukocytes in cervical cancer samples are lymphocytes, then neutrophils, and in lower frequency macrophages. We have also observed a significant increase in the frequency of low density CD66b+ cells in the PBMC fraction of cancer patients compared to patients with precursor lesions or controls (Alvarez et al, 2017). In parallel, recent research in the laboratory has shown that in PBMCs of cancer patients there is an increase in the expression of phospho-STAT3 and decrease in the expression of phospho-p65 NF kappa-B. Using a HPV associated murine tumor model, we have observed that inhibition of STAT3 leads to significant increase in expression of phospho-p65 NF kappa-B expression in the spleen (Figure 1) and significant increase in anti-tumor immune responses, with the consequent inhibition in tumor growth. Importantly, the effects of inhibition of STAT3 were partially reversed by co-treatment with inhibitor of NF kappa-B (Rossetti et al, 2020).
Interestingly, using an in vitro model, where we co-culture macrophages with tumor cells spheroids, we have also showed that inhibition of lactate production by tumor cells, modulates the phenotype of tumor associated macrophages, reducing the HIF-1 and increasing p65 NF kappa-B expression, and also increasing the antigen presentation capacity (Stone et al, 2019).Therefore, interfering with STAT3 and NF kappa-B signaling may be used as tools for therapy against HPV associated tumors.
Besides soluble mediators, extracellular vesicles display several effects on the immune system. These vesicles are secreted by several types of cells, including, tumor cells. We have shown that monocytes and B lymphocytes uptake tumor borne extracellular vesicles (Figure 2), and we have also shown that these vesicles can modulate cytokine secretion by monocytes and inhibit T cell proliferation. We are currently studying the mechanisms involved in these observations.
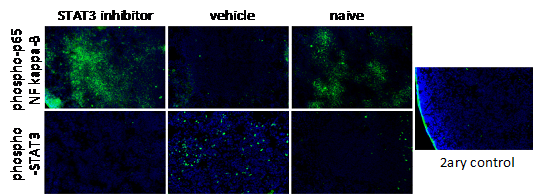
Figure 1. Increase in NF kappa-B expression upon STAT3 inhibition. Mice with TC-1 tumors (cells expressing E6 and E7 from HPV16) were treated with 5 g/Kg NCS74589, STAT3 inhibitor, daily for 7 to 8 days, after tumors were palpable. Harvested spleens were frozen in Tissue-Tek OCT, sectioned and used for immunofluorescence assays for detection of phospho-p65 NF kappa-B and phospho-STAT3 (in green). As controls, we used spleens from naïve mice and sections incubated only with secondary antibody (2ary control).
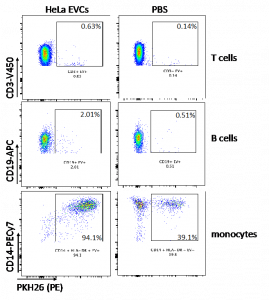
Figure 2. HeLa secreted extracellular vesicles (EVCs) were uptaken by monocytes (CD14) and B lymphocytes (CD19). Vesicles were labeled with PKH26 before incubation with peripheral blood mononuclear cells for 24 hours. Cells were then labeled with the indicated antibodies and analyzed by flow cytometry.
The Best Team
Publicações
The interaction of SET and protein phosphatase 2A as target for cancer therapy. Dacol EC, Wang S, Chen Y, Lepique AP. Biochim Biophys Acta Rev Cancer. 2021 Aug;1876(1):188578. doi: 10.1016/j.bbcan.2021.188578.
Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms. Herbster S, Trombetta-Lima M, de Souza-Santos PT, Paladino A, Silveira CRF, Sogayar MC, Villa LL, Lepique AP, Boccardo E. Cancers (Basel). 2021 May 6;13(9):2217. doi: 10.3390/cancers13092217.
The Role of Autophagy in Tumor Immunology-Complex Mechanisms That May Be Explored Therapeutically. de Souza ASC, Gonçalves LB, Lepique AP, de Araujo-Souza PS. Front Oncol. 2020 Dec 1;10:603661. doi: 10.3389/fonc.2020.603661.
Local and Systemic STAT3 and p65 NF-KappaB Expression as Progression Markers and Functional Targets for Patients With Cervical Cancer. Rossetti RAM, da Silva-Junior IA, Rodríguez GR, Alvarez KLF, Stone SC, Cipelli M, Silveira CRF, Beldi MC, Mota GR, Margarido PFR, Baracat EC, Uno M, Villa LL, Carvalho JP, Yokochi K, Rosa MBSF, Lorenzi NP, Lepique AP. Front Oncol. 2020 Nov 19;10:587132. doi: 10.3389/fonc.2020.587132.
Stone SC, Rossetti RAM, Alvarez KLF, Carvalho JP, Margarido PFR, Baracat EC, Tacla M, Boccardo E, Yokochi K, Lorenzi NP, Lepique AP. Lactate secreted by cervical cancer cells modulates macrophage phenotype. J Leukoc Biol. 2019 May;105(5):1041-1054.
Rossetti RAM, Lorenzi NPC, Yokochi K, Rosa MBSF, Benevides L, Margarido PFR, Baracat EC, Carvalho JP, Villa LL, Lepique AP. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses. PLoS One. 2018;13:e0199034.
da Silva-Junior IA, Dalmaso B, Herbster S, Lepique AP, Jancar S. Platelet-Activating Factor Receptor Ligands Protect Tumor Cells from Radiation-Induced Cell Death. Front Oncol. 2018;8:10.
da Silva Junior IA, Stone SC, Rossetti RM, Jancar S, Lepique AP. Modulation of Tumor-Associated Macrophages (TAM) Phenotype by Platelet-Activating Factor (PAF) Receptor. J Immunol Res. 2017;2017:5482768.
Alvarez KLF, Beldi M, Sarmanho F, Rossetti RAM, Silveira CRF, Mota GR, Andreoli MA, Caruso EDC, Kamillos MF, Souza AM, Mastrocalla H, Clavijo-Salomon MA, Barbuto JAM, Lorenzi NP, Longatto-Filho A, Baracat E, Lopez RVM, Villa LL, Tacla M, Lepique AP. Local and systemic immunomodulatory mechanisms triggered by Human Papillomavirus transformed cells: a potential role for G-CSF and neutrophils. Sci Rep. 2017;7:9002.
da Silva IA Jr, Chammas R, Lepique AP, Jancar S. Platelet-activating factor (PAF) receptor as a promising target for cancer cell repopulation after radiotherapy. Oncogenesis. 2017;6:e296.
The role of inflammation in HPV carcinogenesis. Boccardo E, Lepique AP, Villa LL. Carcinogenesis. 2010;31:1905-12.
Interleukin-10 production by tumor infiltrating macrophages plays a role in Human Papillomavirus 16 tumor growth. Bolpetti A, Silva JS, Villa LL, Lepique AP. BMC Immunol. 2010;11:27.
HPV16 tumor associated macrophages suppress antitumor T cell responses. Lepique AP, Daghastanli KR, Cuccovia IM, Villa LL. Clin Cancer Res. 2009;15:4391-400.
HPV vaccination: the beginning of the end of cervical cancer? – A Review. Lepique AP, Rabachini T, Villa LL. Mem Inst Oswaldo Cruz. 2009;104:1-10.
c-Myc protein is stabilized by fibroblast growth factor 2 and destabilized by ACTH to control cell cycle in mouse Y1 adrenocortical cells. Lepique AP, Moraes MS, Rocha KM, Eichler CB, Hajj GN, Schwindt TT, Armelin HA. J Mol Endocrinol. 2004;33:623-38.
Characterization of vascular adhesion molecules that may facilitate progenitor homing in the post-natal mouse thymus. Lepique AP, Palencia S, Irjala H, Petrie HT. Clin Dev Immunol. 2003;10:27-33.
Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. Plotkin J, Prockop SE, Lepique A, Petrie HT. J Immunol. 2003;171:4521-7.
Copyright © 2025 Programa de Pós-Graduação em Imunologia | Produzido por SCS - Mídias Online


