Denise Morais da Fonseca, PhD
Head of the Laboratory of Mucosal Immunology Laboratory
Telephone: +55 (11) 2648 8415
E-mail: denisefonseca@usp.br
Lattes: http://lattes.cnpq.br/1717770435586287
Google Scholar: https://scholar.google.com.br/citations?user=Iax_C2oAAAAJ&hl=en
Research Lines:
Gut-lung axis: understanding the immune dialogue between barrier tissues in the development of diseases
Mucosal and barrier tissues are endowed with a specialized immune system and specific effector mechanisms designed to respond to an enormous range of regional challenges while maintaining tissue homeostasis. In this sense, specialized cells and immune mediators are found in each mucosal tissue. Classically, subtypes of dendritic cells, T lymphocytes, and antibody-producing B cells are differentially regulated in the mucosa. Our research group has been working to identify non-classical mechanisms acting not only in maintaining tissue homeostasis but also in the communication between barrier tissues, such as the intestine and the lung. The importance of the gut-lung axis in disease control and promotion has been studied in different contexts, focusing on mechanisms involving cell trafficking, changes in the microbiota composition and microbial metabolites. In this context, at the Mucosal Immunology Laboratory, we study how episodes of infection affecting the structure of the mesentery and lymphatics impact in host immunity and metabolism, and in the integration of the immune response between the different barrier tissues. We believe that the mechanisms mediating the immunological communication between the intestinal and pulmonary mucosa have a confluence point in the mesentery. To understand the mechanisms involved in the immune dialogue between mucosal tissues, particularly the gut and lung, we study the functional impact of infectious and non-infectious environmental challenges in the gut for lung immunity, focusing on the involvement of the microbiota and its metabolites, adjacent adipose tissue compartments and lymphatic vessels.
‘Immunological scar’ after intestinal infection in the development of metabolic disorders: study of the interactions between the microbiota and the immune system of the mesentery
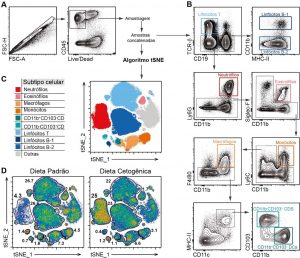
During the last century, changes in population’s lifestyles, including improvements in health conditions, use of vaccines, antibiotics, nutritional supplements and balanced diets, have not only improved quality of life, but also changed the way the host responds to pathogenic challenges. Despite this, episodes of acute infection are still quite common throughout the world, particularly those that affect areas of the body that are more exposed to the environment, such as the intestinal mucosa. By using experimental models of acute infection that affect mucosal tissues, we study how such infectious episodes can have long-term immunological and metabolic consequences for the host. Defined episodes of infection can leave an “immunological scar” in the mesentery characterized by the mesenteric and lymphatic (Figure 1), chronic inflammation of the mesenteric adipose tissue (Figure 2) and aberrant traffic of gut-derived dendritic cells (Figure 3). As a consequence of these events, there is induction of immunity against components of the intestinal microbiota, susceptibility to the development of inflammatory bowel diseases and metabolic disorders. At the Laboratory of Mucosal Immunology, we study the interactions between microbiota – immune system – nervous system that determine the development of the “immunological scar” in the mesentery.
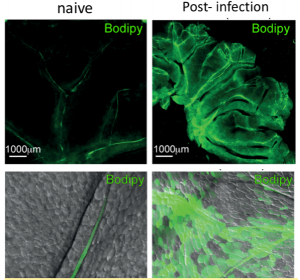
Figure 1: Identification of mouse mesenteric lymphatic vessels after Bodipy FL C16 gavage (in green) in naive animals or after gastrointestinal infection with the bacterium Yersinia pseudotuberculosis.
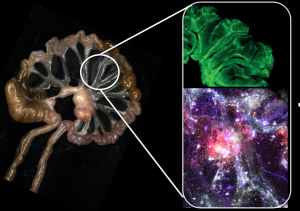
Figure 2: ‘Immunological Scar’ developed by mice previously infected with the bacterium Yersinia pseudotuberculosis. Left image represents the intestine, mesentery, and post-infection mesenteric lymph nodes. On the right, upper image shows the lesion of the mesenteric lymphatic vessels (in green) and the lower image shows inflammatory infiltrate in the post-infection mesentery (green:CX3CR1, blue:CD11b, red: CD45, white CD11c).
Mechanisms associated with the integrity and repair of mesenteric lymphatic vessels
The mesenteric lymphatic vessels play essential function in the absorption of vitamins and lipids from the diet and, at the same time, serve as pathways for the traffic of gut-derived dendritic cells that carry intestinal antigens to be presented to T lymphocytes in the mesenteric lymph nodes. This process ensures immunological communication in the intestinal mucosa. External factors such as infection and dietary changes can interfere with the integrity and function of these lymphatic vessels. In this project, we study the mechanisms associated with permanent or transient increase in lymphatic permeability during infectious or inflammatory processes (such as in colitis and cancer) in the intestine or during dietary changes.
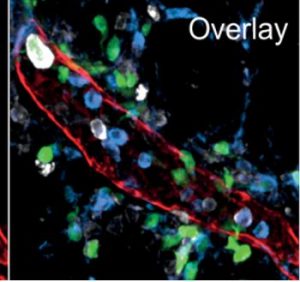
Figure 3: Structural detail of a mesenteric lymphatic vessel and associated myeloid cells. Red:Lyve1, green:CD11c, blue:CD11b, white: MHC II.
Effect of dietary changes on the microbiota and on the immune system associated with intestinal and pulmonary mucosa
The intestinal mucosa is the largest tissue in the human body exposed to the external environment. Different factors act directly on the intestine and interfere with the homeostasis of the immune system cells associated with the intestine. Such factors include infections, stress and diet changes. Recent studies have shown alterations in the homeostasis of the intestine-associated immune system, as well as in the intestinal microbiota, reflect on distal tissues, particularly other mucosal tissues. Although some individuals need certain dietary restrictions due to metabolic or immune disorders, most people who undergo dietary changes do so in an unsupervised manner. Little is known about the long-term effects of dietary changes on the immune system associated with the intestine and other mucous membranes. In this project we study how dietary changes including the use of ketogenic (low carb), low protein, hyperlipidic, fructose-rich or hypercholesterolemic diets interfere in the function of the immune system associated with the intestine and lung.
Contribution of the omentum to the immunity of the intestinal mucosa
Different cellular and molecular components act for immunity in barrier tissues. Such components include immune system cells, epithelia, antimicrobial peptides, antibodies and the microbiota itself. Such mechanisms promote selective physical segregation between host and external environment and provide protection against the entrance of pathogens and, at the same time, prevent the elaboration of responses against innocuous antigens (such as food, allergens and microbiota). Recently, visceral adipose tissue depots have been described as a very important component of the intestinal mucosal barrier. White adipose tissue is home to a wide variety of immune system cells, including memory cells specific for gut antigens. This project aims to understand the contribution of the omentum to immune effector mechanisms in the intestinal mucosa. We intend to study how infection by different types of pathogens, bacteria, viruses or protozoa interferes in the structure of lymphatic vessels in the omentum, as well as in the dynamics of activation of immune system cells residing in the omentum.
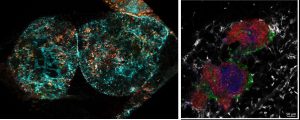
Figure 5: Immunological structures present in the omentum. Left, lymphatic vessel remodeling (in cyan) after intestinal infection. On the right, cell cluster (milky spot) composed of B lymphocytes (red and green), T (blue) and macrophages (white).
Recent Publication
Fonseca DM, Hand TW, Han SJ, Gerner MY, Byrd AL, Harrison OJ, Quinones M, Trinchieri G, Brodsky IE, Germain RN, Randolph GJ, Belkaid Y. Microbiota-dependent sequelae of acute infection compromises tissue-specific immunity. Cell. 2015 Oct 8;163(2):354-66.
Bhattacharjee A, Burr AHP, Overacre-Delgoffe AE, Tometich JT, Yang D, Huckestein BR, Linehan JL, Spencer SP, Hall JA, Harrison OJ, Fonseca DM,Belkaid Y, Hand TW. Enteropathy-induced regulatory T cells inhibit intestinal CD4+ T 1 cell responses against oral vaccines. Immunity, 2021.
de Campos Fraga-Silva TF, Mimura LAN, de Oliveira LRC, Dos Santos Toledo JH, Borim PA, Zorzella-Pezavento SFG, Alonso DP, Ribolla PEM, de Oliveira CAF, da Fonseca DM, Villablanca EJ, Sartori A. Selenization of S. cerevisiae increases its protective potential in experimental autoimmune encephalomyelitis by triggering an intestinal immunomodulatory loop. Sci Rep. 2020.
Nascimento D, Viacava PR, Ferreira RG, Damaceno MA, Piñeros AR, Melo PH, Donate PB, Toller-Kawahisa JE, Zoppi D, Veras FP, Peres RS, Menezes-Silva L, Caetité D, Oliveira AER, Castro IMS, Kauffenstein G, Nakaya HI, Borges MC, Zamboni DS, Fonseca DM, Paschoal JAR, Cunha TM, Quesniaux V, Linden J, Queíroz CunhaF, Ryffel B, Alves-Filho JC. Sepsis expands a CD39 + plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity. Immunity, 2021.
Menezes-Silva L, Fonseca DM**. Connecting the dots in type 1 diabetes: The role for gut-pancreas axis. J Leukoc Biol. 2019
Henrique MO, Neto LS, Assis JB, Barros MS, Capurro ML, Lepique AP, Fonseca DM**, Sá-Nunes A**. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019
Nunes FPB, Alberca-Custódio RW, Gomes E, Fonseca DM, Yokoyama NH, Labrada A, Russo M. TLR9 agonist adsorbed to alum adjuvant prevents asthma-like responses induced by Blomia tropicalis mite extract. J Leukoc Biol. 2019
Fachi JL, Felipe JS, Pral LP, da Silva BK, Corrêa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de Sales E Souza ÉL, Dos Santos Martins F, Guima SES, Thomas AM, Setubal JC, Magalhães YT, Forti FL, Candreva T, Rodrigues HG, de Jesus MB, Consonni SR, Farias ADS, Varga-Weisz P, Vinolo MAR. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Reports. 2019.
Han SJ, Zaretsky AG, Andrade-Oliveira V, Collins N, Fonseca DM, Dzutsev A, Shaik J, Harrison OJ, Tamoutounour S, Byrd AL, Smelkinson M, Bouladoux N, Bliska J, Brenchley JM, Brodsky IE, Belkaid Y. The white adipose tissue is a hub for memory T cells and promotes memory responses. Immunity, 2017.
Piñeros AR, Campos LW, Fonseca DM, Bertolini TB, Gembre AF, Prado RQ, Alves-Filho JC, Ramos, SG, Russo M, Bonato VLD. M2 macrophages or IL-33 treatment attenuate ongoing Mycobacterium tuberculosis infection. Sci Rep. 2017 in press.
Wowk PF, Franco LH, Fonseca DM, Paula MO, Vianna ÉD, Wendling AP, Augusto VM, Elói-Santos SM, Teixeira-Carvalho A, Silva FD, Vinhas SA, Martins-Filho OA, Palaci M, Silva CL, Bonato VL. Mycobacterial Hsp65 antigen upregulates the cellular immune response of healthy individuals compared to tuberculosis patients. Hum Vaccin Immunother. 2017
Munhoz TD, Anai LA, Fonseca DM, Semolin LM, Sueiro FR, Tinucci-Costa M. Regulatory T cells in dogs with multicentric lymphoma: peripheral blood quantification at diagnosis and after initial stage chemotherapy. Arquivo Brasileiro de Medicina Veterinária e Zootecnia (Online), v. 68, p. 1-9, 2016.
Edwards, Justin P. ; Hand, Timothy W. ; Da Fonseca, Denise Morais ; Glass, Deborah D. ; Belkaid, Yasmine ; Shevach, Ethan M. . The GARP/Latent TGF-β1 complex on Treg cells modulates the induction of peripherally derived Treg cells during oral tolerance. European Journal of Immunology, v. in, p. in press, 2016.
Askenase MH, Han SJ, Byrd AL, Fonseca DM, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su XZ, Trinchieri G, Grainger JR, Belkaid Y. Bone marrow resident NK cells prime monocytes for regulatory function during infection. Immunity. 2015 Jun 16;42(6):1130-42.
Fonseca DM, Wowk PF, Paula MO, Gembre AF, Turato W, Campos LW, Silva CL, Ramos SG, Horn C, Marchal G, Arruda KL, Russo M, Bonato VLD. Requirement of MyD88 and Fas pathways for the efficacy of experimental allergen-free therapy. Allergy. 2015 Mar;70(3):275-84.
Grainger JR, Askenase MH, Guimont-Desrochers F, Fonseca DM, Belkaid Y. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol Rev. 2014 May;259(1):75-87. doi: 10.1111/imr.12167.
Faustino L, Fonseca DM, Florsheim EB, Resende RR, Lepique AP, Faquim-Mauro E, Gomes E, Silva JS, Yagita H, Russo M. Tumor necrosis factor-related apoptosis-inducing ligand mediates the resolution of allergic airway inflammation induced by chronic allergen inhalation. Mucosal Immunol. 2014 Feb 26. doi: 10.1038/mi.2014.9.
Faustino L, da Fonseca DM, Takenaka MC, Mirotti L, Florsheim EB, Guereschi MG, Silva JS, Basso AS, Russo M. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol. 2013 Mar 15;190(6):2614-21. doi: 10.4049/jimmunol.Lucas
Fonseca DM, Wowk PF, Paula MO, Campos LW, Gembre AF, Turato WM, Ramos SG, Dias-Baruffi M, Barboza R, Gomes E, Silva CL, Russo M, Bonato VL. Recombinant DNA immunotherapy ameliorate established airway allergy in a IL-10 dependent pathway. Clin Exp Allergy. 2012 Jan;42(1):131-43.
Fonseca DM, Paula MO, Wowk PF, Campos LW, Gembre AF, Turato WM, Ramos SG, Dias-Baruffi M, Barboza R, Gomes E, Horn C, Marchal G, Arruda LK, Russo M, Bonato VL. IFN-γ-mediated efficacy of allergen-free immunotherapy using mycobacterial antigens and CpG-ODN. Immunol Cell Biol. 2011 Oct;89(7):777-85.
Fonseca DM, Trombone AP, Repeke CE, Avila-Campos MJ, Coelho-Castelo AA, Silva JS, Campanelli AP, Deperon Bonato VL, Garlet GP. Functional interferences in host inflammatory immune response by airway allergic inflammation restrain experimental periodontitis development in mice. J Clin Periodontol. 2011 Feb;38(2):131-41.
Morais Fonseca D, Rosada RS, e Paula MO, Wowk PF, Franco LH, Soares EG, Silva CL, Deperon Bonato VL. Experimental tuberculosis: designing a better model to test vaccines against tuberculosis. Tuberculosis (Edinb). 2010 Mar;90(2):135-42.
Fonseca DM, Silva CL, Wowk PF, Paula MO, Ramos SG, Horn C, Marchal G, Bonato VL. Mycobacterium tuberculosis culture filtrate proteins plus CpG Oligodeoxynucleotides confer protection to Mycobacterium bovis BCG-primed mice by inhibiting interleukin-4 secretion. Infect Immun. 2009 Dec;77(12):5311-21.
Fonseca DM, Silva CL, Paula MO, Soares EG, Marchal G, Horn C, Bonato VL. Increased levels of interferon-gamma primed by culture filtrate proteins antigen and CpG-ODN immunization do not confer significant protection against Mycobacterium tuberculosis infection. Immunology. 2007 Aug;121(4):508-17.
Copyright © 2025 Programa de Pós-Graduação em Imunologia | Produzido por SCS - Mídias Online